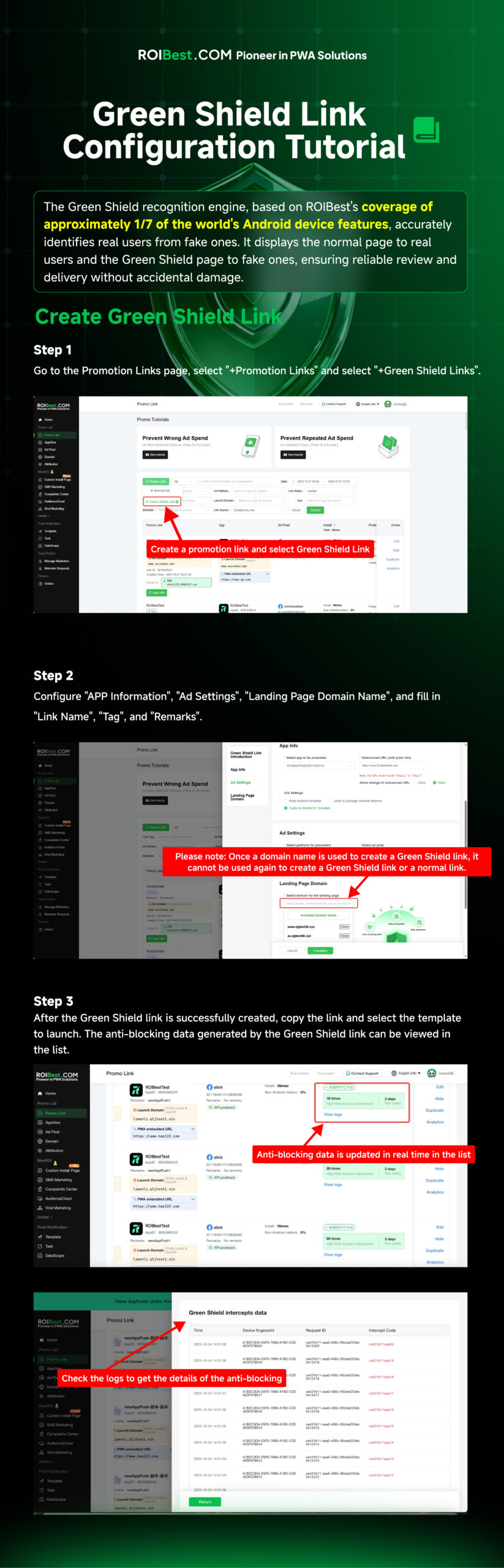
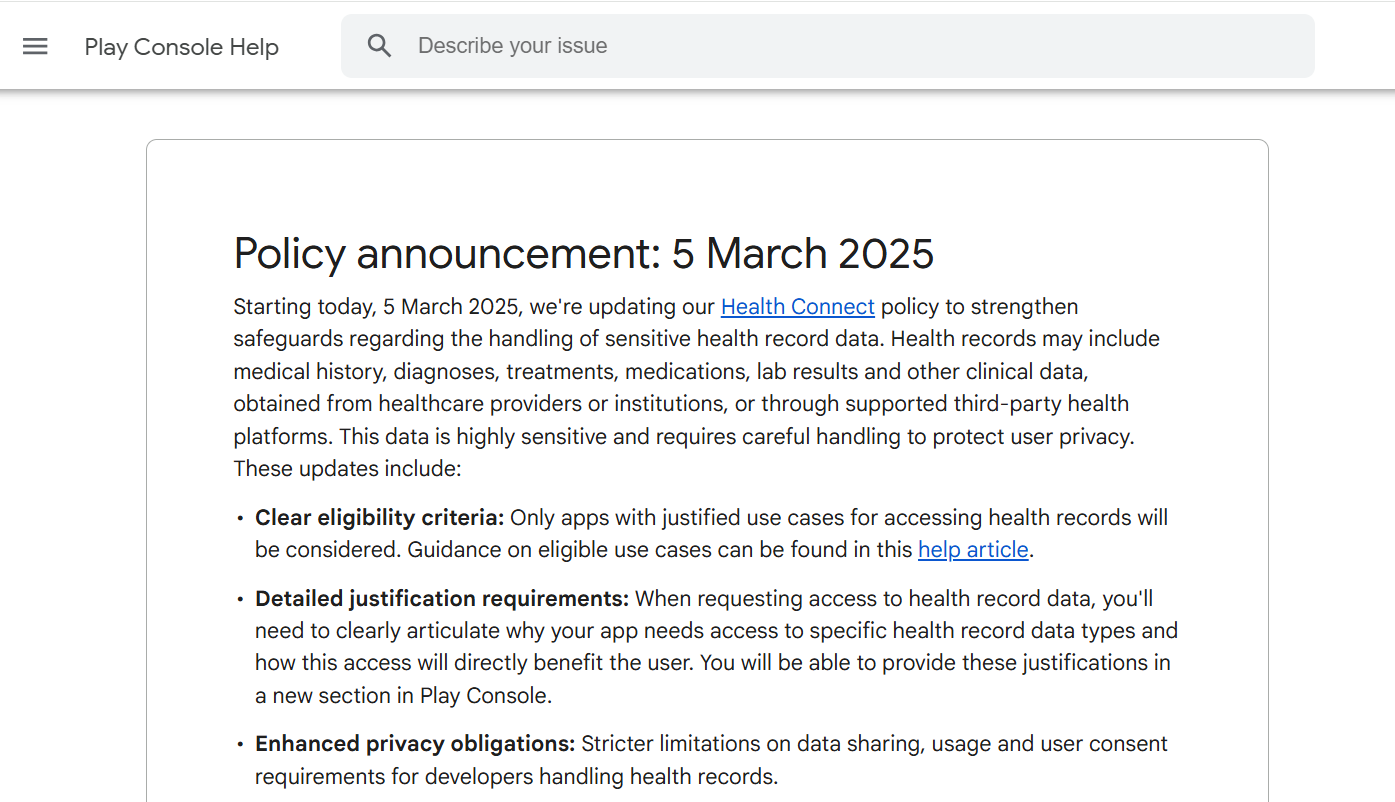
These years, global regulations such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) have all imposed strict rules on health-related apps, requiring explicit user consent, strong data protection, and tight access controls. These regulations significantly raise the compliance threshold, driving up development costs and forcing apps to adopt robust privacy-by-design practices from the start. Google is aligning these new requirements with best practices and current global regulatory expectations, to protect users and reduce potential health misinformation. For small developers, they could face may face higher costs and slower release cycles, while clinically validated apps could gain competitive advantage.
-
Apps may now require legal review and medical compliance checks before publishing or updating, making the release cycles even slower.
-
The risk of app rejection or removal increases if disclosures are unclear or insufficient, making pre-submission audits essential.
-
Developers will face higher legal, medical, and UX design overhead to comply.
Overall, the update may improve user trust but also accelerates market consolidation around well-resourced players.